Medical breakthrough: Seattle man is first adult to get new gene therapy for vision
Toby Willis gave up on medical miracles long ago.
The Seattle man’s eyesight started deteriorating when he was just a kid and couldn’t figure out why he kept crashing into things. By the time he was in his early 40s, Willis’ visual world had dimmed to shadows and silhouettes.
Still, he refused to obsess over scientific advances.
“I made it a point not to go down those rabbit holes, to be chasing that pipe dream,” Willis said.
Nevertheless, the possibility that something might be on the horizon occasionally tickled at the back of his mind.
So Willis checked in with his eye doctor late last year, setting in motion a train of events that culminated March 20 at Children’s Hospital Los Angeles (CHLA) when he became the first adult to undergo the first gene therapy approved in the U.S. for treatment of an inherited disease.
The medication, called Luxturna, is injected in the eye and uses a harmless virus to deliver working versions of a gene to the cells of patients whose own genes are defective. It targets a single, rare mutation, but has raised hopes that after decades of disaster and disappointment, a wave of gene therapies for other disorders is on the way.
At the same time, Luxturna’s staggering price tag of $425,000 per eye is raising questions about who can afford the newest medical technologies — and whether the results will justify the costs.
Willis already knows it won’t be a miracle cure for him. The therapy is expected to work best in children diagnosed early, before disease causes irreversible damage to their eyes. In Willis’ case, doctors hope it will keep the 44-year-old software engineer’s eyesight from getting worse and — perhaps — improve his ability to distinguish objects and navigate the world.
“As much as I would love to have a full range of 20-20 vision, that’s not realistic for me,” Willis said. “But to be able to recognize my fiancee’s face or see my guide dog, or maybe enjoy a more rich experience in my travels and hiking — that would be a big deal.”
Without the surgery, Willis’ disease would eventually extinguish his eyesight altogether.
For physicians who have helplessly watched patients like Willis go blind, even an imperfect treatment represents a breakthrough. For gene-therapy researchers, it’s a thrilling validation.
“I think this is a game changer,” said Timothy Cherry, who’s exploring different ways to repair inherited forms of blindness at Seattle Children’s Research Institute. “What I see as inspiring is that this shows us that the fundamental idea works.”
Resourceful patientWillis grew up in rural Tennessee, where he played and worked on his family’s farm. By middle school he had to sit at the front of the class to see the board. Doctors diagnosed retinitis pigmentosa, a range of disorders that relentlessly erode the biological machinery of sight.
His mother, a nurse, launched her son on a crash course in Blindness 101: mastering Braille, learning to navigate blindfolded, practicing with a cane.
When Willis encountered job discrimination, he started his own industrial maintenance business.
“People were scared to hire me because my eyes didn’t work perfectly,” he said. “It forced me to be entrepreneurial and forge my own ways.”
Seeking greater independence, a progressive community and better mass transit, he moved to Seattle in 2006 with his first guide dog at his side.
In his new home, Willis created a nonprofit called Independence Guide Dogs, to train animals and match them with owners. His long-standing fascination with computer technology and frustration with the roadblocks it presents to people with disabilities landed him a job at Expedia, where he works to make the online travel-booking site accessible to all.
The company’s generous health benefits covered the cost of having both eyes treated with Luxturna. But first, Willis had to get tested in Seattle to find out if his vision loss was caused by a defective version of a gene called RPE65, which is vital for recycling molecules used to convert light into the electrical signals the brain processes into images.
The geneticist was giddy when she called to tell Willis he carried the mutation found in only about 1,000 to 2,000 Americans, knowing it meant he might benefit from the new therapy. The disorder caused by the mutation is called Leber congenital amaurosis, a more precise diagnosis than Willis got as a teen in Tennessee.
Just seven medical centers in the country are approved by Luxturna’s developer, Spark Therapeutics, to deliver the treatment. Willis picked The Vision Center at CHLA, which offers the therapy to children and adults, and headed there as soon as possible.
“My doctor said, ‘Time is retinal cells.’ The longer I wait, the more I have to lose,” he said.
His left-eye surgery came a few hours after 13-year-old Jack Hogan in Boston became the country’s first Luxturna patient outside of clinical trials. Willis’ right eye was treated on March 27.
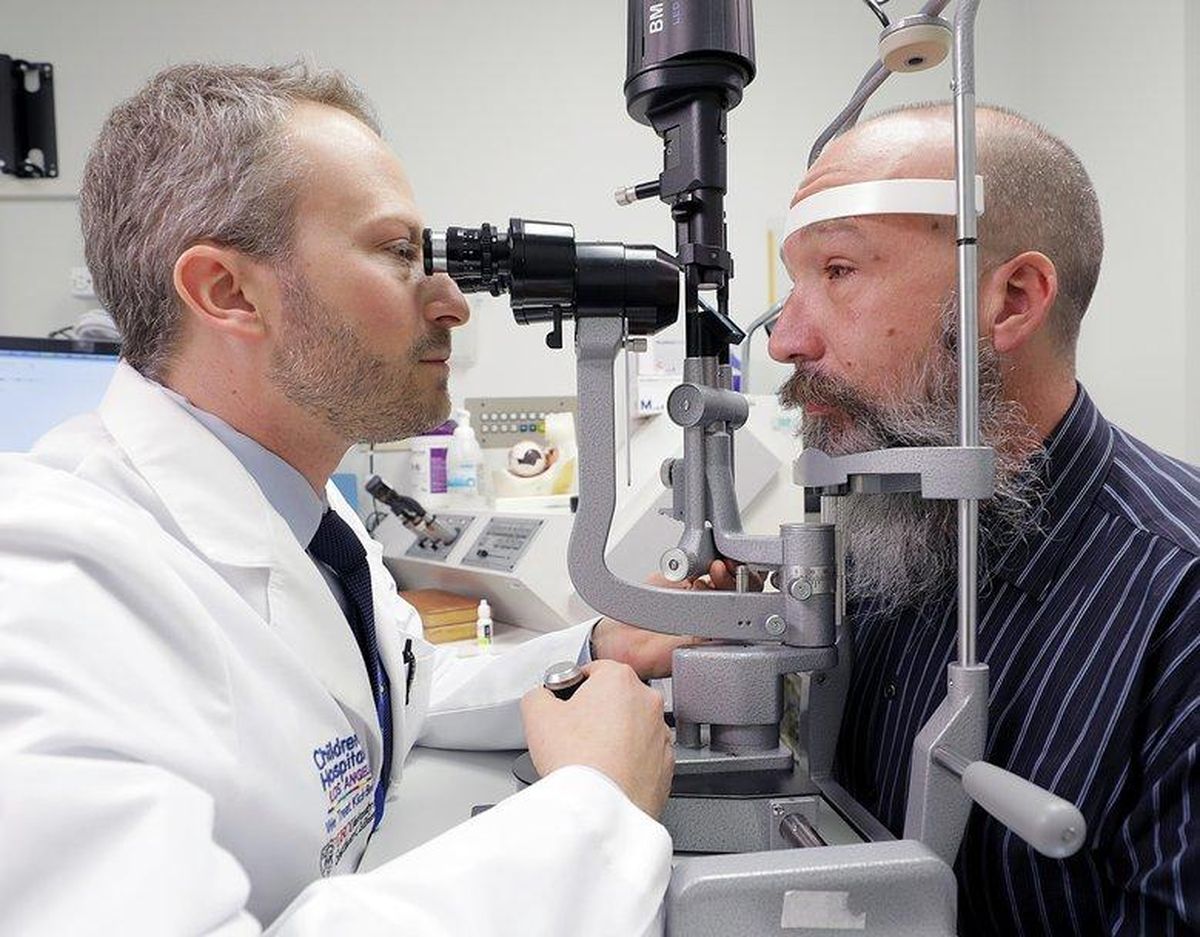
Exacting procedureThe procedure takes only about an hour, but the level of precision required is nerve-wracking, said CHLA Vision Center surgeon and researcher Dr. Aaron Nagiel. With a tiny light illuminating the inside of the eyeball, he had to thread a needle through an inserted port and inject a .03 milliliter drop of viral solution between the photosensitive rod-and-cone layer of the retina and an underlying layer, called the retinal pigment epithelium, where the RPE65 gene carries out its mission.
If something goes wrong, there’s a single backup vial of the costly fluid.
Each injection contains about 150 billion viruses, which invade the target cells and deposit “good” genes into their nuclei.
In clinical trials, 27 out of 29 patients experienced improvements in eyesight that lasted at least a year, and more than 70 percent were able to navigate better in very low light. Some participants experienced dramatic revelations, like seeing the stars for the first time. One patient’s vision got worse.
The treatment can trigger cataracts, but that’s not a problem for Willis. Cataracts are common in people with his condition, possibly triggered by inflammation, so he already had his cloudy lenses replaced with clear, synthetic versions.
“In some ways I’m the perfect patient,” he said. “I’ve lost enough vision that the risk-benefit ratio is much more tilted toward the benefit side.”
The eye also presents a “perfect storm” of favorable conditions for the first gene-therapy success after nearly 30 years of failed attempts, Cherry said.
The entire field of research was nearly derailed in 1999, when a runaway immune response killed an 18-year-old man in a trial treatment for a liver disorder. In 2003, an experimental gene therapy for the immune deficiency called “bubble boy” syndrome activated cancer-causing genes and triggered leukemia in two children.
Immune response is muted in the eye, so there’s little chance the gene-carrying virus will trigger rejection, Cherry explained. And because the virus used in Luxturna doesn’t insert its genes directly into the host cell’s chromosomes, the danger of switching on disease genes is low.
The eye is also a small target, which means a small drug dose. And decades of basic research on the causes of blindness laid a solid foundation for the breakthrough by identifying culprit genes and figuring out how they work.
Gene therapies for several other inherited diseases are already in the pipeline, including against sickle cell disease and a type of muscular dystrophy. Common eye disorders, like glaucoma, diabetic retinopathy and macular degeneration, will be tougher to tackle because they involve multiple genes as well as lifestyle factors, Cherry cautioned.
“We have high hopes that we can push on these sorts of technologies and deliver more treatments in the future, but it’s important to be realistic,” he said.
It’s still wait and seeDays after his first treatment, Willis said he already noticed that his world seemed brighter, as if more light was getting through. Edges appeared a little crisper.
The full benefits won’t be clear for another month, and Willis is trying to keep his hopes in check.
Even if all the treatment does is stop further deterioration, Willis says that would make it worthwhile to him.
“This is really the only treatment we have for this devastating, progressive form of blindness,” Nagiel said.
But one of the biggest uncertainties is how long the benefits will last.
The latest data from Sparks Therapeutics, which hasn’t yet been published, extends out three years.
The mother of a 5-year-old boy with the same genetic mutation recently discussed the treatment with Nagiel. Her main worry is that her son would be devastated if his eyesight cleared, only to be lost again after a few years.
“I had never thought of that,” Nagiel said. “But it’s a concern … and we don’t have the answers yet.”