GU student using lasers and LEGOs in quest for stable hydrogen production
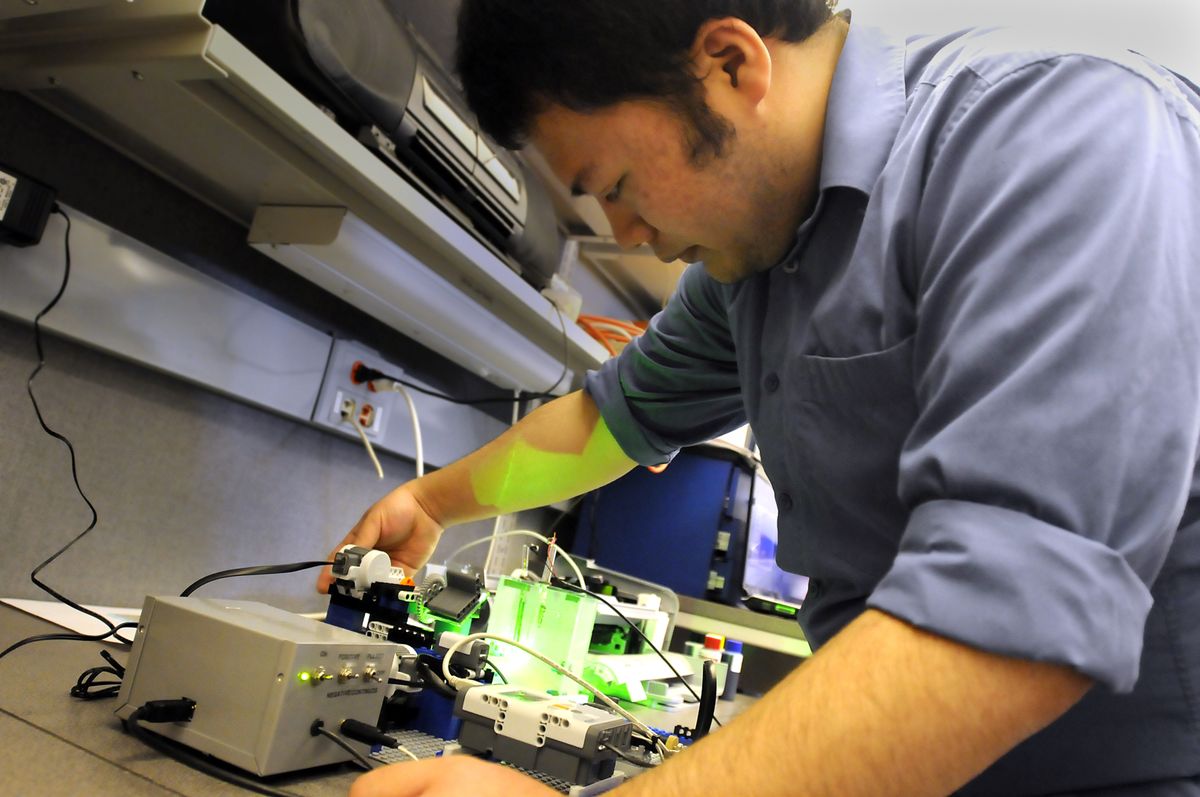
Some day, an undergraduate with a LEGO set may save the planet by finding a cheap way to produce hydrogen from water, using sunlight.
It could be Thanh Do, an international student studying chemistry and math at Gonzaga University.
After all, Do and research assistants like him at eight other undergraduate institutions nationwide have a head start on those of us whose main work with LEGOs involves stepping on our kids’ pieces on the way to the bathroom in the middle of the night.
“Our approach is relatively easy to explain to someone even with a minimal technical background,” wrote Bruce Parkinson, a chemistry professor at the University of Wyoming and creator of the project that he hopes will find the perfect combination of “semiconducting metal oxides to photoelectrolyze water.”
It has been more than 30 years since Japanese scientists Akira Fujishma and Kenichi Honda found that titanium dioxide exposed to light caused water to separate into hydrogen and oxygen.
But since then, nobody has found an affordable, stable and efficient catalyst to allow the process to compete with hydrocarbons as a fuel source. When someone does, it will mean an unlimited source of energy that does not release carbon into the atmosphere.
“It’s all about the catalyst,” said David Cleary, Do’s professor of chemistry at Gonzaga.
But there are a lot of metals on the periodic chart, and finding the right combination of just four of them would take millions of tries.
So Parkinson came up with the idea of dividing the research into smaller pieces, letting undergraduate students do the work. To start, nine colleges were chosen to work out any problems with the kits he and his graduate students designed to test the waters, so to speak.
To be practical, the kits had to be inexpensive and easy to put together.
Here’s where the LEGO Mindstorm Kits – which Parkinson’s kids used to play with – come in.
A LEGO motor manipulates the beam from a small laser pointer, which simulates sunlight acting upon the metal oxide catalyst, mixed in different combinations.
Do and the other research assistants use an ordinary Hewlett-Packard printer to “ink jet” the catalyst onto a glass slide and then bake the slide in a laboratory oven at 500 degrees. Each pixel on the slide represents a different combination of the metals.
Do is experimenting with iron, cesium and chrome – three metals that together have more than 34,000 possible combinations. The slide is immersed into a water-filled glass cell, and the laser illuminates it one pixel at a time, seven hours per slide. A computer analysis reveals where on the slide the greatest release of hydrogen occurred.
“Some of the catalysts we have come up with work, but not for very long,” Cleary said.
When Do or someone else finds one that remains stable, it will be put to more serious research, Parkinson said.
“Our dream is that the army of enthusiastic young researchers will not only come up with improvements in the screening techniques but also discover promising new materials that will make the inexpensive direct solar photoelectrolysis of water a reality and move us closer to a hydrogen economy based on renewable resources,” Parkinson wrote in his application for the research grant.
The project is funded through the Camille and Henry Dreyfus Foundation and the National Science Foundation.