Guidant deal imperiled
INDIANAPOLIS — Johnson & Johnson indicated Wednesday it might cancel its planned $25.4 billion acquisition of struggling medical device maker Guidant Corp. even as federal regulators gave conditional approval for the deal.
Guidant officials countered that J&J is legally obligated to complete the deal that was announced last December.
Guidant shares sank more than 4.4 percent, dropping $2.77 to $60.33 in afternoon trading on the New York Stock Exchange where it had fallen to a new 52-week low of $59.25 earlier in the day. J&J shares lost 42 cents to $61.48.
J&J said recent recalls of Guidant-made implantable devices and regulatory investigations at Guidant have had a “material adverse effect” on the Indianapolis-based company and that it was not required to close the acquisition.
New Brunswick, N.J.-based J&J said it was talking with Guidant about restructuring terms of the proposed buyout but that no agreement had been reached.
“Johnson & Johnson cannot assure that the companies will resume those discussions or, if discussions do resume, whether they will be able to reach agreement on revised terms that would allow Johnson & Johnson to proceed with the transaction,” company officials said in a statement.
Pulling out of the agreement could cost J&J $700 million, according to the company’s merger agreement.
But Guidant’s chief executive Ronald W. Dollens said the acquisition still makes sense.
“Recent product and communications issues have certainly had an impact on our business in the near term,” he said in a statement. “However, we believe that the fundamentals of our business are strong and our markets and products have attractive prospects for growth.”
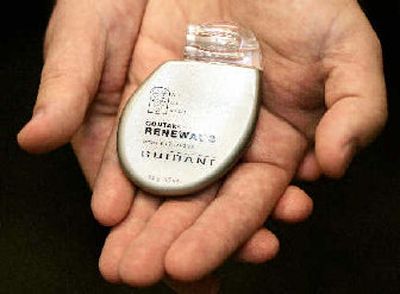
Since June, Guidant has recalled or issued warnings about 88,000 heart defibrillators — including its top seller, the Contak Renewal 3 — and almost 200,000 pacemakers because of reported malfunctions. The company faces multiple lawsuits from patients and shareholders, as well as a reported criminal investigation by the U.S. Food and Drug Administration.
Last week, federal prosecutors in Boston and Minneapolis issued separate subpoenas seeking documents about Guidant’s devices and its Ventak Prizm R 2 and Contak Renewal R 1 and 2 defibrillators.
The J&J-Guidant deal was announced in December but was subject to regulatory approval because of antitrust concerns.
The FTC said Wednesday that J&J would have to divest devices used in bypass graft surgery and allow another company to license certain drug-eluting stent technology to avoid violating federal laws regulating competition with the Guidant deal. The agency said J&J would also have to end its agreement to distribute certain products used in heart surgery.